ISO 13485:2016 provides the quality requirements for the medical device management sector for regulatory purposes.
The implementation of ISO 13485:2016 is essential for companies that develop, manufacture and distribute medical equipment and that want to demonstrate their competitive ability and performance on national and international markets. ISO 13485:2016 is the standard most requested by medical device manufacturers, internationally (in the USA, Japan, Canada, the European Union).
ISO 13485:2016, as a specific standard of the medical device quality system, complements the ISO 9001:2015 standard. Additional requirements relate to model and process control – including environmental control, special processes, traceability, record keeping and corrective action – which are more stringent for the medical device industry.
Advantages of ISO 13485:2016 certification:
- Risk minimization, their control
- More effective, transparent internal processes
- Meeting of customer requirements, as well as other stakeholders (employees, suppliers, investors)
- Obtaining a competitive advantage
- Cost reduction
- Improving the overall performance and capabilities of the organization
- Compliance with the legal provisions in force
The certification process
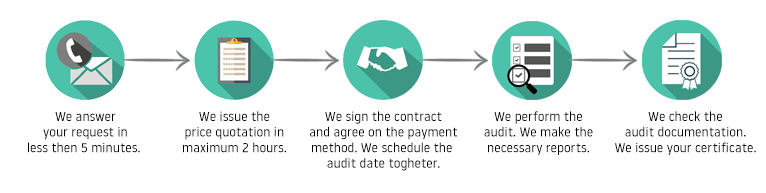
Certification costs
The price of a certification according to ISO 13485:2016 is established depending on the structure, size and field of activity of the organization.

